Notes for Page 49.
We followed the following PowerPoints during class:
1) Intro to Ionic Compounds
2) Properties of Ionic Compounds
We used this note sheet.
Thursday, October 27, 2011
Ionic Compounds
Labels:
2nd 9 weeks,
Anion,
Cation,
Ionic Compounds,
Ions,
Metals,
Non-Metals
Scientific Notation
Notes for page 35.
Scientists work with very large and very small numbers. writing these nubmers over and over gets repetitive, boring and time consuming. Therefore, scientists developed amn easier way to write these numbers.
Scientific notation is based on multples of ten.
- positive exponents are multpilying by ten
* the number gets larger
* The deciman moves to the right
- negative exponents are dividing by ten
* the number gets smaller
* The decimal moves to the left
Integrating Significant Figures
- the decimal point always goes behind the first non-zero
- only include the significant figures when converting to scientific notation
- once in scientific notation, only the numbers are significant (NOT the "x 10")
Scientists work with very large and very small numbers. writing these nubmers over and over gets repetitive, boring and time consuming. Therefore, scientists developed amn easier way to write these numbers.
Scientific notation is based on multples of ten.
- positive exponents are multpilying by ten
* the number gets larger
* The deciman moves to the right
- negative exponents are dividing by ten
* the number gets smaller
* The decimal moves to the left
Integrating Significant Figures
- the decimal point always goes behind the first non-zero
- only include the significant figures when converting to scientific notation
- once in scientific notation, only the numbers are significant (NOT the "x 10")
Friday, October 7, 2011
Lewis Dot Structures
Notes for page 37.
Bohr Diagrams show ALL electrons.
Lewis Dot Structures show only the electrons in the last orbital of the Bohr Diagram.
These electrons are called Valence Electrons.
Valence Electrons
1) Find the element on the PT
2) Write down the element's symbol
3) Find the element's Group #
4) Draw Valence Electrons around the symbol (starting at the top, moving clockwise, one at a time until all sides are filled, then pair)
How Group # corresponds to Valence Electron #

Bohr Diagrams show ALL electrons.
Lewis Dot Structures show only the electrons in the last orbital of the Bohr Diagram.
These electrons are called Valence Electrons.
Valence Electrons
- Electrons in the outermost orbital of an atom
- The reactive electrons
- Determine an element's physical and chemical properties
- Found by looking at Group # on the PT (excluding Transition Metals)
- Show # of valence electrons
- Show bonding between atoms
1) Find the element on the PT
2) Write down the element's symbol
3) Find the element's Group #
4) Draw Valence Electrons around the symbol (starting at the top, moving clockwise, one at a time until all sides are filled, then pair)
How Group # corresponds to Valence Electron #

Labels:
1st 9 Weeks,
Atom,
Groups,
Lewis Dot Structures,
Valence Electrons
Tuesday, September 20, 2011
Light and Planck's Constant
Notes for Page 29.
Review: (all Review Points taken from Page 17)
1) Energy of electrons increases as you move away from the nucleus.
2) You cannot pinpoint the exact location of an electron - only the general area (Heisenburg Uncertainty Principle - loosely)
3) Bohr put electrons in orbitals.
Waves:
Frequency is inversly proportional to wavelength
- As frequency increases, wavelength decreases (gets shorter)
- As frequency decreases, wavelength increases (gets longer)
The formula for the relationship between frequency and wavelength is:
Websites:
Click Here for an explanation of quanta, or photon.
Click Here for an explanation of Planck's Constant.
Click Here for the history and explanation of Planck's Constant.
Review: (all Review Points taken from Page 17)
1) Energy of electrons increases as you move away from the nucleus.
2) You cannot pinpoint the exact location of an electron - only the general area (Heisenburg Uncertainty Principle - loosely)
3) Bohr put electrons in orbitals.
Waves:
Frequency is inversly proportional to wavelength
- As frequency increases, wavelength decreases (gets shorter)
- As frequency decreases, wavelength increases (gets longer)
The formula for the relationship between frequency and wavelength is:
Why do I care about waves?
1) Light travels in waves
2) Electrons are made of light
Light
- Travels in waves
- Very fast
- Electromagnetic Spectrum
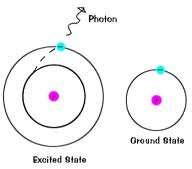
How does this apply to Chemistry?
- Electrons are photons of light
- Atoms have an emission spectrum!
* called the Atomic Emission Spectrum
* Seen when an excited atom passes through a gas
How does it work?
1) Atom absorbs a certain amount of energy
3) Moves back down to its normal energy level (Ground State)
- emits a packet of energy (quanta or photon) as it does this
4) We can see this emission of energy
- comes out as color!
- Metals heat up and change color
- Max Planck wanted to explain this change of color
* determined that energy changes in set units (quanta)
- Planck's Constant
* Pertains to amount of energy released when an excited electron goes back to its Ground State
* States: The amount of radiant energy (E) absorbed or emitted is proportional to the frequency of the
radiation absorbed.
Planck's Constant = 6.6262 x 10 ^-34 Js
Websites:
Click Here for an explanation of quanta, or photon.
Click Here for an explanation of Planck's Constant.
Click Here for the history and explanation of Planck's Constant.
Labels:
1st 9 Weeks,
Electrons,
Excited Electron,
Light,
Photoelectric Effect,
Quanta,
Unit 2,
Waves
Monday, September 19, 2011
Bohr Diagram
Things you need to know before you can draw Bohr Structures:
Atoms contain protons, neutrons, and electrons.
Protons
Bohr Structure
 electrons. (n = orbital #)
electrons. (n = orbital #)
Atoms contain protons, neutrons, and electrons.
Protons
- Positively charged
- Located in the Nucleus
- Contribute to Mass
- Contribute to Charge
- Tell you which element you have
- # CANNOT change
- Are = to the Atomic Number
- Neutrally charged
- Located in the Nucleus
- Contribute to mass
- # CAN change (isotopes)
- Negatively charged
- Located in the orbitals
- No mass
- Contribute to charge
- # CAN change
Bohr Structure
- Shows ALL electrons
- Maps electrons in their orbitals
 electrons. (n = orbital #)
electrons. (n = orbital #)- Orbital #1 can hold 2 electrons.
- Orbital #2 can hold 8 electrons.
- Orbital #3 can hold 18 electrons.
- Orbital #4 can hold 32 electrons.
- Ask yourself "How many protons are there in this neutral atom?"
- Based on Mass #, determine number of nuetrons.
- Since it's a neutral atom: # p = # e
- Draw orbitals (# orbitals = Period #)
- Place electrons in orbitals
- Orbital #1 - electrons pair together at the top of the circle
- All other Orbitals - electrons are place one at a time starting at the top, moving clockwise
- Once you have four electrons in an orbital the electrons start pairing up.
- Once you reach the max number that an orbital can hold you move to the next orbital.
Labels:
1st 9 Weeks,
Atom,
Atomic Number,
Electrons,
Orbitals,
Periodic Table,
Protons,
Unit 2
Friday, September 16, 2011
Average Atomic Mass
Every element has at least 2 naturally occurring isotopes.
This begs the question, "If elements can have different masses, why is there only one mass on the Periodic Table?"
The mass on the Periodic Table is actually the Average Atomic Mass of all of that element's isotopes.
Quick notes on Average Atomic Mass:

This is what the formula means:
Carbon has 2 main isotopes: Carbon-12 and Carbon-13.
C-12 makes up about 98% of all of the Carbon in the world.
C-13 makes up about 1.1% of all of the Carbon in the world.
To find the Average Atomic Mass of Carbon:
1) Change % to decimal
98% = .98
1.1% = .011
2) Multiply decimal by corresponding mass
.98 x 12 = 11.76 amu
.011 x 13 = .143 amu
3) Add the products together
11.76 amu + .143 amu = 11.903 amu
4) The Average Atomic Mass of Carbon is 11.903 amu
Websites
Here is a video explaining how to calculate Average Atomic Mass.
This website gives a brief explanation and a few examples.
Worksheets
This is a great worksheet on Average Atomic Mass.
This begs the question, "If elements can have different masses, why is there only one mass on the Periodic Table?"
The mass on the Periodic Table is actually the Average Atomic Mass of all of that element's isotopes.
Quick notes on Average Atomic Mass:
- Units: amu (Atomic Mass Unit)
- The mass on the PT
- Comprised of the average of all isotopic masses

This is what the formula means:
- Isotopes don't exist in equal amounts
- Take each isotope's mass
- Multiply it by its % abundance (in decimal form)
- Do this for all isotopes of the element
- Add them together
Carbon has 2 main isotopes: Carbon-12 and Carbon-13.
C-12 makes up about 98% of all of the Carbon in the world.
C-13 makes up about 1.1% of all of the Carbon in the world.
To find the Average Atomic Mass of Carbon:
1) Change % to decimal
98% = .98
1.1% = .011
2) Multiply decimal by corresponding mass
.98 x 12 = 11.76 amu
.011 x 13 = .143 amu
3) Add the products together
11.76 amu + .143 amu = 11.903 amu
4) The Average Atomic Mass of Carbon is 11.903 amu
Websites
Here is a video explaining how to calculate Average Atomic Mass.
This website gives a brief explanation and a few examples.
Worksheets
This is a great worksheet on Average Atomic Mass.
Labels:
1st 9 Weeks,
Atomic Mass,
Isotopes,
Periodic Table,
Unit 2
Extra Credit Opportunity #2
Extra Credit Opportunity
Due: Monday, September 19
Create Flashcards of the following:
1. Scientists involved in Atomic Theory Development (7)
Front: Name of Scientist
Back: Year, contribution, experiment
2. Scientists involved in the Development of the Modern Periodic
Table (6)
Front: Name of Scientist
Back: How he arranged the PT, what he contributed
3. Ions (2)
Front: Type of Ion
Back: Definition, what causes ions to form
4. Isotopes (1)
Front: Isotope
Back: Definition, what causes isotopes to form
5. Subatomic Particles (3)
Front: Name of subatomic particle
Back: Charge, mass, location
6. Families (4)
Front: Family Name
Back: Group #
Due: Monday, September 19
Create Flashcards of the following:
1. Scientists involved in Atomic Theory Development (7)
Front: Name of Scientist
Back: Year, contribution, experiment
2. Scientists involved in the Development of the Modern Periodic
Table (6)
Front: Name of Scientist
Back: How he arranged the PT, what he contributed
3. Ions (2)
Front: Type of Ion
Back: Definition, what causes ions to form
4. Isotopes (1)
Front: Isotope
Back: Definition, what causes isotopes to form
5. Subatomic Particles (3)
Front: Name of subatomic particle
Back: Charge, mass, location
6. Families (4)
Front: Family Name
Back: Group #
Thursday, September 15, 2011
Isotopes and Ions
Notes for Page 23.
Atoms are identified by their # of protons.
Atoms are identified by their # of protons.
- The # of protons DOES NOT change
- The # of neutrons CAN change
- The # of electrons CAN change
When the # of neutrons and electrons change the atomic structure/description also changes.
Isotopes
- Atoms of the SAME element with DIFFERENT atomic masses
- Occur when a change in neutrons occurs
- All elements have at least two naturally occurring elements
Two ways to write isotopes
1)
Ions
- Atoms with a charge
- Occur when there is a change in # of electrons
Two types:
1) Cation - positively charged ion
2) Anion - negatively charged ion
Charge is written in the top right corner of the chemical symbol
Quizzes
Quiz #1 is a great quiz!
Quiz #2 is another great quiz!
Quiz #3 is a very basic matching quiz.
Quiz #4 is a really great quiz.
Worksheets
This is a great worksheet on the Subatomic Chart that includes Isotopes and Ions.
This is a great worksheet on atomic structure with isotopes and ions.
PT Review and Intro to Atomic Structure
Notes for Page 21.
In neutral (n) atoms
Proton # is = to Electron #.
In all atoms
Mass # is = p + n
Atoms
- Smallest unit of an element that retains elemental properites
(Dalton's Postulates)
- Are made of Subatomic particles (sub = smaller than, atomic = atom: smaller than an atom)
1) Protons
* found in the nucleus
* charge of +1
* mass = 1 amu
2) Neutrons
* found in the nucleus
* no charge (neutral)
* mass = 1 amu
3) Electrons
* found in the electron cloud
* charge of -1
* negligible (no) mass
In neutral (n) atoms
Proton # is = to Electron #.
In all atoms
Mass # is = p + n
Atoms
- Smallest unit of an element that retains elemental properites
(Dalton's Postulates)
- Are made of Subatomic particles (sub = smaller than, atomic = atom: smaller than an atom)
1) Protons
* found in the nucleus
* charge of +1
* mass = 1 amu
2) Neutrons
* found in the nucleus
* no charge (neutral)
* mass = 1 amu
3) Electrons
* found in the electron cloud
* charge of -1
* negligible (no) mass
Wednesday, September 14, 2011
History of the Periodic Table
Notes for Page 19.
Click here to download the PowerPoint presentation from class.
If you are unable to download the PowerPoint, please email me at EORoark713@gmail.com or at Elizabeth.Roark@aliefisd.net.
*Please allow 24 hours for a response.
Click here to download the PowerPoint presentation from class.
If you are unable to download the PowerPoint, please email me at EORoark713@gmail.com or at Elizabeth.Roark@aliefisd.net.
*Please allow 24 hours for a response.
Tuesday, September 13, 2011
History of the Atomic Theory
Notes for Page 17.
Click here to download the PowerPoint presentation from class.
If you are unable to download, please email me at EORoark713@gmail.com or at Elizabeth.Roark@aliefisd.net.
*Please allow 24 hours for a response.
Click here to download the PowerPoint presentation from class.
If you are unable to download, please email me at EORoark713@gmail.com or at Elizabeth.Roark@aliefisd.net.
*Please allow 24 hours for a response.
Monday, September 12, 2011
Accuracy vs. Precision
Notes for Page 15.
Science is based on measurement.
Scientists test the same measurement over and over.
Accuracy
Closeness to the True Value
When a measurement equals the True Value
True Value is what you expect to get.
It is also referred to as the Accepted Value or the Given Value.
Ex.
Hits the Bulls eye
Precision
Degree of reproduce-ability
When you get the same number over and over.
You can be precise, but not accurate.
You can be accurate, but not precise.
You can be precise and accurate.
Websites
This website has explanations as well as examples.
Science is based on measurement.
Scientists test the same measurement over and over.
Accuracy
Closeness to the True Value
When a measurement equals the True Value
True Value is what you expect to get.
It is also referred to as the Accepted Value or the Given Value.
Ex.
Hits the Bulls eye
Precision
Degree of reproduce-ability
When you get the same number over and over.
You can be precise, but not accurate.
You can be accurate, but not precise.
You can be precise and accurate.
Websites
This website has explanations as well as examples.
Sunday, September 11, 2011
The Periodic Table of Elements
Notes for Page 13
Chemistry hinges on The Periodic Table.
Chemistry hinges on The Periodic Table.
This is the Periodic Table that will be given to you when you take TAKS. (click to view a larger picture)
 You can find multiple things on this Periodic Table (PT from here on out).
You can find multiple things on this Periodic Table (PT from here on out).1) At the top is a picture of the element Silicon. In this picture, TAKS shows you where the Atomic Number, Atomic Mass, Symbol, and Name can be found.
Atomic Number = # of protons
Atomic Mass = average atomic mass of the element, # protons + # neutrons
2) Across the top of the PT are Roman Numerals IA-VIIIA. These are called Groups and will help you find valence electron number and oxidation number.
3) Down the PT are numbers 1-7. These are referred to as Periods and tell you how many orbitals an element has.
4) There is a bold line that looks like a "staircase." Along this line are the elements Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, and Polonium. These are Metalloids.
5) To the left of the metalloids are metals. To the right of the metalloids are non-metals.
6) The elements in Group 1 are the Alkali Metals.
7) The elements in Group 2 are the Alkaline Earth Metals.
8) The elements in Groups 3-12 are the Transition Metals.
9) The elements in Group 17 are the Halogens.
10) The elements in Group 18 are the Noble Gases.
Websites
This is a great website that shows the PT according to names of groups and allows you to click on individual elements.
Labels:
1st 9 Weeks,
Groups,
Metalloids,
Metals,
Non-Metals,
Oxidation Numbers,
Periodic Table,
Periods,
Valence Electrons
Thursday, September 1, 2011
Significant Figures
Scientists use varying pieces of equipment. Different pieces of equipment measure to a different degree of preciseness.
For example : One scale might measure to the hundredths place (.12) while another scale might measure to the thousandths (.120).
We have to account for this when we add, subtract, multiply, or divide numbers of varying preciseness.
We do this by using Significant Figures.
Rules:
1. Any non-zero number (1-9) is significant.
Ex.
12 has 2 significant figures: 12
- both 1 and 2 are non-zeroes
12.3 has 3 significant figures: 12.3
- 1, 2, and 3 are non-zeroes
2. Any zero between a non-zero is significant.
Ex.
101 has 3 significant figures: 101
- 1 is significant (Rule #1)
- the zero is between the ones
1002 has 4 significant figures: 1001
- 1 and 2 are significant (Rule #1)
- the 2 zeroes between them are significant
3. Any zero before the first non-zero is not significant.
Ex.
0.102 has 3 significant figures: 0.102
- 1 and 2 are significant (Rule #1)
- the zero between the 1 and 2 is significant (Rule #2)
- The first zero is not significant because it comes before the first non-zero.
0.00012 has 2 significant figures: 0.00012
- 1 and 2 are significant (Rule #1)
- All of the zeroes are before the first non-zero and are not significant
4. Any zero after a non-zero with a decimal point is significant.
Ex.
1.0 has 2 sig figs: 1.0
- the 1 is significant (Rule #1)
- the 0 is significant because there is a decimal
1.2030 has 5 sig figs: 1.2030
- the 1,2, and 3 are significant (Rule #1)
- the first zero is between two non-zeros and is significant (Rule #2)
- the last zero is after the last non-zero and there is a decimal point.
5. Any zero after a non-zero without a decimal point is not significant.
Ex.
10 only has 1 sig fig: 10
- 1 is significant (Rule #1)
- there is no decimal point
1010 has 3 sig figs: 1010
- 1 is significant (Rule #1)
- the first zero is between two non-zeros (Rule #2)
- the last zero is not accompanied by a decimal and is therefore not significant.
Adding and Subtracting
When you add or subtract numbers the answer should be rounded to the lowest number of decimal places.
Steps:
1) Add or subtract as the problem states.
12.03 + 1.9 = 13.93
2) Identify # of decimal places
12.03 + 1.9 = 13.93Ex.
2 dp 1dp
3) Identify lowest number of decimal places
1.9 has only 1 decimal place
4) Round answer to match the lowest number of decimal places
Since the lowest number of decimal places is 1 my answer can
only have 1 decimal place.
13.93 is rounded to 13.9.
5) Correct Answer: 13.9
2.5233 - .125874 = 2.397426
4 dp 6dp 2.3974 is the correct answer
35.63 + .2586 = 35.8886
2 dp 4 dp 35.89 is the correct answer
Multiplication and Division
When you multiply or divide numbers the answer should be rounded to the lowest number of significant figures.
Steps:
1) Carry out multiplication or division as usual.
12 x 30 = 360
2) Identify numbers of significant figures.
12 has 2 sig figs (Rule #1)
30 has 1 sig fig (Rule #1 and #5)
3) Identify lowest number of sig figs
30 has 1 sig fig which is the lowest number of sig figs
4) Round the answer to the lowest number of sig figs.
360 has 2 sig figs (Rule #1 and #5) and will round to 400 to match 1 sig
fig.
5) Correct Answer: 400
Ex.
30560 x 101 = 3086560
4 sig figs 3 sig figs 3090000 is the correct answer
(R #1,2,5) (R #1,2)
65 / 200 = 0.325
2 sf 1 sf 0.3 is the correct answer
(R#1) (R#1,5) *Note that the 2 and 5 are not turned to 0s like in the first example. These zeroes
would be significant based on Rule #4 and would give the answer 3 sig figs.
Websites:
Replacement Assignment print this out and turn it in on Tuesday, September 6. If you are using it to replace Significant Figures Homework on Page 10 it must be turned in on Tuesday. If you are using it as extra credit you have until Friday.
Click here for an awesome explanation of rules with great examples.
For example : One scale might measure to the hundredths place (.12) while another scale might measure to the thousandths (.120).
We have to account for this when we add, subtract, multiply, or divide numbers of varying preciseness.
We do this by using Significant Figures.
Rules:
1. Any non-zero number (1-9) is significant.
Ex.
12 has 2 significant figures: 12
- both 1 and 2 are non-zeroes
12.3 has 3 significant figures: 12.3
- 1, 2, and 3 are non-zeroes
2. Any zero between a non-zero is significant.
Ex.
101 has 3 significant figures: 101
- 1 is significant (Rule #1)
- the zero is between the ones
1002 has 4 significant figures: 1001
- 1 and 2 are significant (Rule #1)
- the 2 zeroes between them are significant
3. Any zero before the first non-zero is not significant.
Ex.
0.102 has 3 significant figures: 0.102
- 1 and 2 are significant (Rule #1)
- the zero between the 1 and 2 is significant (Rule #2)
- The first zero is not significant because it comes before the first non-zero.
0.00012 has 2 significant figures: 0.00012
- 1 and 2 are significant (Rule #1)
- All of the zeroes are before the first non-zero and are not significant
4. Any zero after a non-zero with a decimal point is significant.
Ex.
1.0 has 2 sig figs: 1.0
- the 1 is significant (Rule #1)
- the 0 is significant because there is a decimal
1.2030 has 5 sig figs: 1.2030
- the 1,2, and 3 are significant (Rule #1)
- the first zero is between two non-zeros and is significant (Rule #2)
- the last zero is after the last non-zero and there is a decimal point.
5. Any zero after a non-zero without a decimal point is not significant.
Ex.
10 only has 1 sig fig: 10
- 1 is significant (Rule #1)
- there is no decimal point
1010 has 3 sig figs: 1010
- 1 is significant (Rule #1)
- the first zero is between two non-zeros (Rule #2)
- the last zero is not accompanied by a decimal and is therefore not significant.
Adding and Subtracting
When you add or subtract numbers the answer should be rounded to the lowest number of decimal places.
Steps:
1) Add or subtract as the problem states.
12.03 + 1.9 = 13.93
2) Identify # of decimal places
12.03 + 1.9 = 13.93Ex.
2 dp 1dp
3) Identify lowest number of decimal places
1.9 has only 1 decimal place
4) Round answer to match the lowest number of decimal places
Since the lowest number of decimal places is 1 my answer can
only have 1 decimal place.
13.93 is rounded to 13.9.
5) Correct Answer: 13.9
2.5233 - .125874 = 2.397426
4 dp 6dp 2.3974 is the correct answer
35.63 + .2586 = 35.8886
2 dp 4 dp 35.89 is the correct answer
Multiplication and Division
When you multiply or divide numbers the answer should be rounded to the lowest number of significant figures.
Steps:
1) Carry out multiplication or division as usual.
12 x 30 = 360
2) Identify numbers of significant figures.
12 has 2 sig figs (Rule #1)
30 has 1 sig fig (Rule #1 and #5)
3) Identify lowest number of sig figs
30 has 1 sig fig which is the lowest number of sig figs
4) Round the answer to the lowest number of sig figs.
360 has 2 sig figs (Rule #1 and #5) and will round to 400 to match 1 sig
fig.
5) Correct Answer: 400
Ex.
30560 x 101 = 3086560
4 sig figs 3 sig figs 3090000 is the correct answer
(R #1,2,5) (R #1,2)
65 / 200 = 0.325
2 sf 1 sf 0.3 is the correct answer
(R#1) (R#1,5) *Note that the 2 and 5 are not turned to 0s like in the first example. These zeroes
would be significant based on Rule #4 and would give the answer 3 sig figs.
Websites:
Replacement Assignment print this out and turn it in on Tuesday, September 6. If you are using it to replace Significant Figures Homework on Page 10 it must be turned in on Tuesday. If you are using it as extra credit you have until Friday.
Click here for an awesome explanation of rules with great examples.
Intensive/Extensive
There are two categories of physical properties:
1) Intensive
2) Extensive
Intensive
Intensive properties are properties that are independent of the amount of matter present.
Ex.
Color - your hair remains the same color after you cut it (decrease the amount of matter present)
DENSITY - density is a ratio and does not change if the state of matter remains the same
Odor - 1/2 of a lemon smells like a lemon, 1/4 of a lemon smells like a lemon
Boiling point - 100 mL of water will boil at 100 degrees F, 200 mL of water will boil at 100 degrees F
Freezing point - 100 mL of water will freeze at 0 degrees F, 200 mL of water will freeze at 0 degrees F
Extensive
Properties that depend on the amount of matter present.
Ex.
Measurements - if I cut a 6 inches straw in half (remove matter), it is no longer 6 inches
Mass - if I remove 5 g from a 10 g sample (remove matter), it no longer weighs 10 g
Volume - if I add 10 mL to a 5 mL (add matter), it no longer has a volume of 5 mL
1) Intensive
2) Extensive
Intensive
Intensive properties are properties that are independent of the amount of matter present.
Ex.
Extensive
Properties that depend on the amount of matter present.
Ex.
Monday, August 29, 2011
Matter
Matter can be classified into two main groups:
Mixtures and Substances.
Mixtures
Mixtures and Substances.
Mixtures
- Classified as either homogenous or heterogenous
- Combination of two or more substances
- Each component retains characteristic properties
- Components can be easily separated

Ex: Pepperoni Pizza
- Combination of two or more substances (crust, cheese, sauce, pepperoni)
- Each component retains characteristic properites (you can taste the cheese AND the pepperoni AND the crust...they tastes don't blend together like a cake)
- Components can be easily seperated (if you don't like pepperoni you can peel it off
- Homo - same
- Homogenous solutions look the "same" throughout
- They have a uniform composition
- Consist of more than one phase of matter
- Solute - part of the solution that gets dissolved
- Solvent - part of the solution that does the dissolving
Ex: Kool-Aid
- Made of two phases: 1) Solid - sugar and powder 2) Liquid - water
- Cannot see the solid and liquid seperately, all looks the same
- Solute - Sugar
- Solvent - Water

Heterogenous
- Hetero - different
- You can see the different parts
Ex: T-Bone steak
- Does not all look the same
- You can see the fat, the bone, and the meat
Classified as either an element or a compound.
Element- Elements are pure substances
- Simplest form of Matter
- Elements can be found on the PT
- Elements are written by their Chemical Symbol.
- Contain one capital letter

Ex: Gold
- Gold is a pure substnace
- Gold can be found on the PT
- Gold is written Au
- There is only one capital letter in its symbol
Compounds
- Combination of Elements
- Have two or more elements in their chemical symbol
- Have two capital letters in their symbols
- Elements do not retain characteristic properties
- Not easily separated
Ex: Salt
- Salt is a combination of the elements Sodium and Chlorine
- Salt is written NaCl
- There are two capitol letters.
- Sodium and Chlorine blend together to create a compound with new chemical properties
Thursday, August 25, 2011
Chemical and Physical Changes
There are two types of change that matter can undergo:
1) Chemical
2) Physical
Chemical Change
Any change that results in the formation of new chemical substances.
Key Words: reacts, forms, rusts, decomposes, digests, burns, forms, releases a gas, acid, cook
Ex:
Physical Change
Any change that doesn't affect the internal structure of molecules. Any change that does not result in the formation of a new substance.
Key Words: cut, break, any phase change (melt, boil, condense, etc) , dissolve
Ex.
Websites:
This website is a quiz on physical and chemical changes.
This website is a short review and a quiz.
This website discusses physical and chemical changes in depth and has links to other content areas of matter.
1) Chemical
2) Physical
Chemical Change
Any change that results in the formation of new chemical substances.
Key Words: reacts, forms, rusts, decomposes, digests, burns, forms, releases a gas, acid, cook
Ex:
- gasoline burning - produces water vapor and CO2
- eggs cooking - proteins uncoil and coil into a new form
- bread rising - yeast converts carbohydrates into CO2 gas
- milk souring - lactic acid is produced
- suntanning - vitamin D and melanin are produced
Physical Change
Any change that doesn't affect the internal structure of molecules. Any change that does not result in the formation of a new substance.
Key Words: cut, break, any phase change (melt, boil, condense, etc) , dissolve
Ex.
- whipping egg whites - air is forced into the fluid, no new substance is produced
- magnetising a compass needle - realigns groups of iron atoms, no new product is produced
- boiling water - heats water, changes its state, does not produce a new product
- dissolving sugar in water - sugar molecules disperse within the water, the individual sugar molecules are unchanged
- dicing potatoes - separates molecules, but you still have potatoes
Websites:
This website is a quiz on physical and chemical changes.
This website is a short review and a quiz.
This website discusses physical and chemical changes in depth and has links to other content areas of matter.
Wednesday, August 24, 2011
Chemical and Physical Properties of Matter
There are two categories that properties of matter can be broken down into:
1) Physical
2) Chemical
Physical Properties
Any characteristic that you can observe without changing the substance that makes up the material
i.e. the appearance.
Ex.
Characteristics of a substance that tell if the substance can undergo a chemical change.
Ex.
Websites
This website has a brief review and quiz.
This website breaks down the differences in a chart. Very informative.
This website has great notes, quizzes, and worksheets.
1) Physical
2) Chemical
Physical Properties
Any characteristic that you can observe without changing the substance that makes up the material
i.e. the appearance.
Ex.
- Shape
- Color
- State of matter
- Measurement
- Odor
- Magnetism - force of attraction or repulsion that acts at a distance (magnets)
- Conductivity - ability to carry an electrical charge
- Malleability - ability to be hammered into sheets
- Ductility - ability to be drawn into wire
- Melting point
- Boiling point
- DENSITY
Characteristics of a substance that tell if the substance can undergo a chemical change.
Ex.
- Combustibility - ability/readiness to combust
- Flammability - ability/readiness to catch fire
- Reactivity to air - reacts to exposure to air
- Reactivity to light - ex. photo film paper
- Acidity (pH)
Websites
This website has a brief review and quiz.
This website breaks down the differences in a chart. Very informative.
This website has great notes, quizzes, and worksheets.
States of Matter
There are 3 main states of matter
1) Solid (s)
2) Liquid (l)
3) Gas (g)
State Changes

Click on image to view larger.
States of Matter
States of matter are described based on 4 things:
Solid
Liquid
Gases


1) Solid (s)
2) Liquid (l)
3) Gas (g)
State Changes

Click on image to view larger.
States of Matter
States of matter are described based on 4 things:
- Particle proximity - how close the particles are to each other
- Compressibility - if the particles can be pushed (compressed) closer to each other
- Definite/non definite shape
- Definite/non definite volume
Solid
- Solid particles are very close together
- Particles are incompressible
- Solids have a definite shape
- Solids have a definite volume
Liquid
- Particles are not as close as those in a solid
- Particles are slightly compressible
- No definite shape
- Do have a definite volume
Gases
- Particles are very far apart
- Particles are very compressible
- No definite shape
- No definite volume

Subscribe to:
Comments (Atom)