Review: (all Review Points taken from Page 17)
1) Energy of electrons increases as you move away from the nucleus.
2) You cannot pinpoint the exact location of an electron - only the general area (Heisenburg Uncertainty Principle - loosely)
3) Bohr put electrons in orbitals.
Waves:
Frequency is inversly proportional to wavelength
- As frequency increases, wavelength decreases (gets shorter)
- As frequency decreases, wavelength increases (gets longer)
The formula for the relationship between frequency and wavelength is:
Why do I care about waves?
1) Light travels in waves
2) Electrons are made of light
Light
- Travels in waves
- Very fast
- Electromagnetic Spectrum
How does this apply to Chemistry?
- Electrons are photons of light
- Atoms have an emission spectrum!
* called the Atomic Emission Spectrum
* Seen when an excited atom passes through a gas
How does it work?
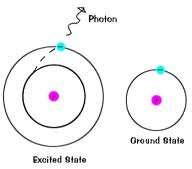
1) Atom absorbs a certain amount of energy
3) Moves back down to its normal energy level (Ground State)
- emits a packet of energy (quanta or photon) as it does this
4) We can see this emission of energy
- comes out as color!
- Metals heat up and change color
- Max Planck wanted to explain this change of color
* determined that energy changes in set units (quanta)
- Planck's Constant
* Pertains to amount of energy released when an excited electron goes back to its Ground State
* States: The amount of radiant energy (E) absorbed or emitted is proportional to the frequency of the
radiation absorbed.
Planck's Constant = 6.6262 x 10 ^-34 Js
Websites:
Click Here for an explanation of quanta, or photon.
Click Here for an explanation of Planck's Constant.
Click Here for the history and explanation of Planck's Constant.






No comments:
Post a Comment