Notes for Page 49.
We followed the following PowerPoints during class:
1) Intro to Ionic Compounds
2) Properties of Ionic Compounds
We used this note sheet.
Chem301
**under construction!**
Thursday, October 27, 2011
Ionic Compounds
Labels:
2nd 9 weeks,
Anion,
Cation,
Ionic Compounds,
Ions,
Metals,
Non-Metals
Scientific Notation
Notes for page 35.
Scientists work with very large and very small numbers. writing these nubmers over and over gets repetitive, boring and time consuming. Therefore, scientists developed amn easier way to write these numbers.
Scientific notation is based on multples of ten.
- positive exponents are multpilying by ten
* the number gets larger
* The deciman moves to the right
- negative exponents are dividing by ten
* the number gets smaller
* The decimal moves to the left
Integrating Significant Figures
- the decimal point always goes behind the first non-zero
- only include the significant figures when converting to scientific notation
- once in scientific notation, only the numbers are significant (NOT the "x 10")
Scientists work with very large and very small numbers. writing these nubmers over and over gets repetitive, boring and time consuming. Therefore, scientists developed amn easier way to write these numbers.
Scientific notation is based on multples of ten.
- positive exponents are multpilying by ten
* the number gets larger
* The deciman moves to the right
- negative exponents are dividing by ten
* the number gets smaller
* The decimal moves to the left
Integrating Significant Figures
- the decimal point always goes behind the first non-zero
- only include the significant figures when converting to scientific notation
- once in scientific notation, only the numbers are significant (NOT the "x 10")
Friday, October 7, 2011
Lewis Dot Structures
Notes for page 37.
Bohr Diagrams show ALL electrons.
Lewis Dot Structures show only the electrons in the last orbital of the Bohr Diagram.
These electrons are called Valence Electrons.
Valence Electrons
1) Find the element on the PT
2) Write down the element's symbol
3) Find the element's Group #
4) Draw Valence Electrons around the symbol (starting at the top, moving clockwise, one at a time until all sides are filled, then pair)
How Group # corresponds to Valence Electron #

Bohr Diagrams show ALL electrons.
Lewis Dot Structures show only the electrons in the last orbital of the Bohr Diagram.
These electrons are called Valence Electrons.
Valence Electrons
- Electrons in the outermost orbital of an atom
- The reactive electrons
- Determine an element's physical and chemical properties
- Found by looking at Group # on the PT (excluding Transition Metals)
- Show # of valence electrons
- Show bonding between atoms
1) Find the element on the PT
2) Write down the element's symbol
3) Find the element's Group #
4) Draw Valence Electrons around the symbol (starting at the top, moving clockwise, one at a time until all sides are filled, then pair)
How Group # corresponds to Valence Electron #

Labels:
1st 9 Weeks,
Atom,
Groups,
Lewis Dot Structures,
Valence Electrons
Tuesday, September 20, 2011
Light and Planck's Constant
Notes for Page 29.
Review: (all Review Points taken from Page 17)
1) Energy of electrons increases as you move away from the nucleus.
2) You cannot pinpoint the exact location of an electron - only the general area (Heisenburg Uncertainty Principle - loosely)
3) Bohr put electrons in orbitals.
Waves:
Frequency is inversly proportional to wavelength
- As frequency increases, wavelength decreases (gets shorter)
- As frequency decreases, wavelength increases (gets longer)
The formula for the relationship between frequency and wavelength is:
Websites:
Click Here for an explanation of quanta, or photon.
Click Here for an explanation of Planck's Constant.
Click Here for the history and explanation of Planck's Constant.
Review: (all Review Points taken from Page 17)
1) Energy of electrons increases as you move away from the nucleus.
2) You cannot pinpoint the exact location of an electron - only the general area (Heisenburg Uncertainty Principle - loosely)
3) Bohr put electrons in orbitals.
Waves:
Frequency is inversly proportional to wavelength
- As frequency increases, wavelength decreases (gets shorter)
- As frequency decreases, wavelength increases (gets longer)
The formula for the relationship between frequency and wavelength is:
Why do I care about waves?
1) Light travels in waves
2) Electrons are made of light
Light
- Travels in waves
- Very fast
- Electromagnetic Spectrum
How does this apply to Chemistry?
- Electrons are photons of light
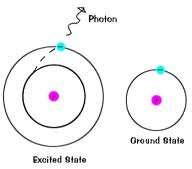
- Atoms have an emission spectrum!
* called the Atomic Emission Spectrum
* Seen when an excited atom passes through a gas
How does it work?
1) Atom absorbs a certain amount of energy
3) Moves back down to its normal energy level (Ground State)
- emits a packet of energy (quanta or photon) as it does this
4) We can see this emission of energy
- comes out as color!
- Metals heat up and change color
- Max Planck wanted to explain this change of color
* determined that energy changes in set units (quanta)
- Planck's Constant
* Pertains to amount of energy released when an excited electron goes back to its Ground State
* States: The amount of radiant energy (E) absorbed or emitted is proportional to the frequency of the
radiation absorbed.
Planck's Constant = 6.6262 x 10 ^-34 Js
Websites:
Click Here for an explanation of quanta, or photon.
Click Here for an explanation of Planck's Constant.
Click Here for the history and explanation of Planck's Constant.
Labels:
1st 9 Weeks,
Electrons,
Excited Electron,
Light,
Photoelectric Effect,
Quanta,
Unit 2,
Waves
Monday, September 19, 2011
Bohr Diagram
Things you need to know before you can draw Bohr Structures:
Atoms contain protons, neutrons, and electrons.
Protons
Bohr Structure
 electrons. (n = orbital #)
electrons. (n = orbital #)
Atoms contain protons, neutrons, and electrons.
Protons
- Positively charged
- Located in the Nucleus
- Contribute to Mass
- Contribute to Charge
- Tell you which element you have
- # CANNOT change
- Are = to the Atomic Number
- Neutrally charged
- Located in the Nucleus
- Contribute to mass
- # CAN change (isotopes)
- Negatively charged
- Located in the orbitals
- No mass
- Contribute to charge
- # CAN change
Bohr Structure
- Shows ALL electrons
- Maps electrons in their orbitals
 electrons. (n = orbital #)
electrons. (n = orbital #)- Orbital #1 can hold 2 electrons.
- Orbital #2 can hold 8 electrons.
- Orbital #3 can hold 18 electrons.
- Orbital #4 can hold 32 electrons.
- Ask yourself "How many protons are there in this neutral atom?"
- Based on Mass #, determine number of nuetrons.
- Since it's a neutral atom: # p = # e
- Draw orbitals (# orbitals = Period #)
- Place electrons in orbitals
- Orbital #1 - electrons pair together at the top of the circle
- All other Orbitals - electrons are place one at a time starting at the top, moving clockwise
- Once you have four electrons in an orbital the electrons start pairing up.
- Once you reach the max number that an orbital can hold you move to the next orbital.
Labels:
1st 9 Weeks,
Atom,
Atomic Number,
Electrons,
Orbitals,
Periodic Table,
Protons,
Unit 2
Friday, September 16, 2011
Average Atomic Mass
Every element has at least 2 naturally occurring isotopes.
This begs the question, "If elements can have different masses, why is there only one mass on the Periodic Table?"
The mass on the Periodic Table is actually the Average Atomic Mass of all of that element's isotopes.
Quick notes on Average Atomic Mass:

This is what the formula means:
Carbon has 2 main isotopes: Carbon-12 and Carbon-13.
C-12 makes up about 98% of all of the Carbon in the world.
C-13 makes up about 1.1% of all of the Carbon in the world.
To find the Average Atomic Mass of Carbon:
1) Change % to decimal
98% = .98
1.1% = .011
2) Multiply decimal by corresponding mass
.98 x 12 = 11.76 amu
.011 x 13 = .143 amu
3) Add the products together
11.76 amu + .143 amu = 11.903 amu
4) The Average Atomic Mass of Carbon is 11.903 amu
Websites
Here is a video explaining how to calculate Average Atomic Mass.
This website gives a brief explanation and a few examples.
Worksheets
This is a great worksheet on Average Atomic Mass.
This begs the question, "If elements can have different masses, why is there only one mass on the Periodic Table?"
The mass on the Periodic Table is actually the Average Atomic Mass of all of that element's isotopes.
Quick notes on Average Atomic Mass:
- Units: amu (Atomic Mass Unit)
- The mass on the PT
- Comprised of the average of all isotopic masses

This is what the formula means:
- Isotopes don't exist in equal amounts
- Take each isotope's mass
- Multiply it by its % abundance (in decimal form)
- Do this for all isotopes of the element
- Add them together
Carbon has 2 main isotopes: Carbon-12 and Carbon-13.
C-12 makes up about 98% of all of the Carbon in the world.
C-13 makes up about 1.1% of all of the Carbon in the world.
To find the Average Atomic Mass of Carbon:
1) Change % to decimal
98% = .98
1.1% = .011
2) Multiply decimal by corresponding mass
.98 x 12 = 11.76 amu
.011 x 13 = .143 amu
3) Add the products together
11.76 amu + .143 amu = 11.903 amu
4) The Average Atomic Mass of Carbon is 11.903 amu
Websites
Here is a video explaining how to calculate Average Atomic Mass.
This website gives a brief explanation and a few examples.
Worksheets
This is a great worksheet on Average Atomic Mass.
Labels:
1st 9 Weeks,
Atomic Mass,
Isotopes,
Periodic Table,
Unit 2
Extra Credit Opportunity #2
Extra Credit Opportunity
Due: Monday, September 19
Create Flashcards of the following:
1. Scientists involved in Atomic Theory Development (7)
Front: Name of Scientist
Back: Year, contribution, experiment
2. Scientists involved in the Development of the Modern Periodic
Table (6)
Front: Name of Scientist
Back: How he arranged the PT, what he contributed
3. Ions (2)
Front: Type of Ion
Back: Definition, what causes ions to form
4. Isotopes (1)
Front: Isotope
Back: Definition, what causes isotopes to form
5. Subatomic Particles (3)
Front: Name of subatomic particle
Back: Charge, mass, location
6. Families (4)
Front: Family Name
Back: Group #
Due: Monday, September 19
Create Flashcards of the following:
1. Scientists involved in Atomic Theory Development (7)
Front: Name of Scientist
Back: Year, contribution, experiment
2. Scientists involved in the Development of the Modern Periodic
Table (6)
Front: Name of Scientist
Back: How he arranged the PT, what he contributed
3. Ions (2)
Front: Type of Ion
Back: Definition, what causes ions to form
4. Isotopes (1)
Front: Isotope
Back: Definition, what causes isotopes to form
5. Subatomic Particles (3)
Front: Name of subatomic particle
Back: Charge, mass, location
6. Families (4)
Front: Family Name
Back: Group #
Subscribe to:
Comments (Atom)